What is HOCl?
HOCl is the scientific formula for hypochlorous acid, a weak acid like that of a mild citrus juice. HOCl is made naturally by white blood cells in all mammals for healing and protection. HOCl is a powerful oxidant that is effective against invading bacteria, fungi, and viruses. HOCl is now used in healthcare, food safety, water treatment, and general sanitation.
How HOCl is produced?
Electrolyzed water is produced by the electrolysis of ordinary tap water containing dissolved sodium chloride (salt). The electrolysis of such salt solutions produces a solution of hypochlorous acid (HClO) and sodium hydroxide (NaOH). The resulting water is a known cleanser and disinfectant. Water electrolysis is discovered in 1970s by Michael Faraday.
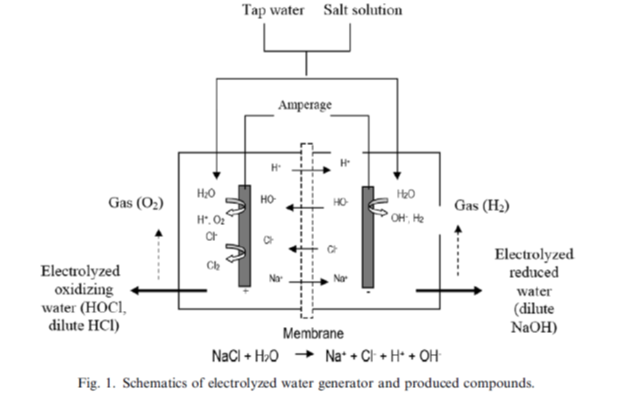
Hypochlorous Acid (HOCl) vs. Hypochlorite (Chlorine Bleach)?
Although they are similar, hypochlorous acid (HOCl) and hypochlorite (OCl-) are still very different. It would be like comparing grape juice to wine.
Hypochlorite ion (OCl) carries a negative electrical charge, while hypochlorous acid (HOCl) carries no electrical charge. The hypochlorous acid moves quickly, able to oxidize the bacteria in a matter of seconds, while the hypochlorite ion might take up to a half hour to do the same. Germ surfaces carry a negative electrical charge which results in a repulsion of the negatively charged hypochlorite ion to the area of the germ surfaces, making hypochlorite ion less effective at killing germs. The hypochlorous acid's lack of electrical charge allows it to more efficiently penetrate the protective barriers surrounding germs.
Hypochlorous acid to be 70-80 times more efficient at killing microbial pathogens than chlorine bleach (OCl-).
Hypochlorous Acid (HOCl) Stability
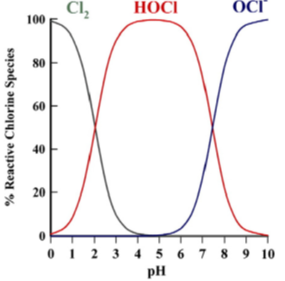
The ratio of the two compounds HOCl and OCl- is determined by the relative acidity (pH) of the water. We can adjust the pH level to make hypochlorous acid more dominate, as it is more efficient at killing bacteria. Hypochlorous acid is a meta-stable molecule. It wants to revert to salt water or convert to hypochlorite. HOCl is most stable under the pH condition of 4.0 to 6.0. At eco-i-on, distilled vinegar is added to lower the pH, keep in the range of pH 5.0 to 5.5. Diluted aqueous solution of hypochlorous acid decomposes very slowly in the dark but more rapidly in the presence of light, particularly rapidly in full sun light, by producing hydrogen chloride and oxygen.
HOCl Regulation